This post is also available in:
 Deutsch
Deutsch
There are a number of checklists available for adhering to quality standards when conducting and reporting a systematic review of the literature. It has been shown that their implementation has led to a more complete reporting (1,2).
Even though most quecklists are meant for reporting, they are worth consulting already when planning and conducting a literature review. We here present some commonly used checklists – depending on your field of work you may consider additional tools as well.
- PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was introduced in its current form in 2009 and has been supplemented with several extensions, e.g. for protocols or scoping reviews (3). A filled in PRISMA checklist and a corresponding flow diagram are expected to be sent in along the manuscript by many journals and are considered standard instruments.
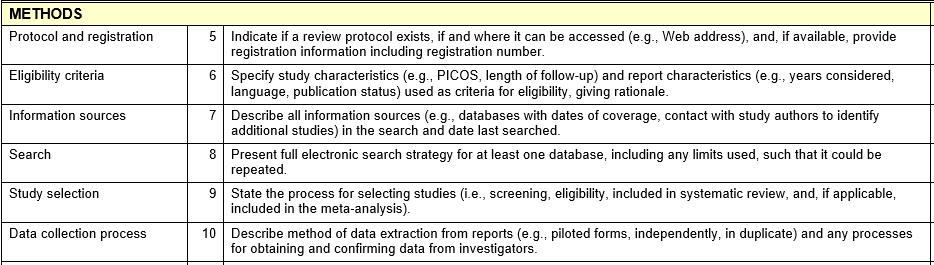
- MECIR (Methodological Expectations of Cochrane Intervention Reviews) with sections for conducting and reporting are methodological standards to which all Cochrane protocols, reviews, and updates must adhere.
- AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews) is a tool for reviewer who have to evaluate the quality of systematic reviews (4). Points in the checklist include e.g. if two researches independently performed the study selection and data extraction or if a satisfactory technique for assessing the risk of bias was used.
- AGREE II (Appraisal of Guidelines for Research and Evaluation) evaluates the process of practice guideline development and the quality of reporting (5).
- MOOSE (Meta-analysis of Observational Studies in Epidemiology) is a reporting checklist for authors and reviewers of meta-analyses of observational studies (6).
- The EQUATOR (Enhancing the Quality and Transparency of Health Research) network website lists reporting guidelines for the main study types.
The main library offers consultations on literature searching and reporting of systematic reviews of the literature.
References
- Tunis AS, McInnes MD, Hanna R, Esmail K. Association of study quality with completeness of reporting: have completeness of reporting and quality of systematic reviews and meta-analyses in major radiology journals changed since publication of the PRISMA statement? Radiology 2013;269(2):413-426.
- Oh JH, Shin WJ, Park S, Chung JS. Reporting and methodologic evaluation of meta-analyses published in the anesthesia literature according to AMSTAR and PRISMA checklists: a preliminary study. Korean J Anesthesiol 2017;70(4):446-455.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6(7):e1000097.
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008.
- Brouwers MC, Kerkvliet K, Spithoff K, on behalf of the AGREE Next Steps Consortium. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ 2016;352:i1152.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker ST, for the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. Meta-analysis of observational studies in epidemiology. A proposal for reporting. JAMA 2000;283(15):2008-2012.
